Moscow region, Odintsovo
st. Soyuznaya, 7
info@techmedmsk.ru
Information about helium cylinders
Information about helium cylinders
In order for more helium to fit into the cylinder, it must first be compressed. Therefore, the gas in the container is under strong pressure. From school physics lessons, it is known that pressure is usually measured in atmospheres (1 atmosphere (atm) is equal to 1 kg / sq. Cm.).
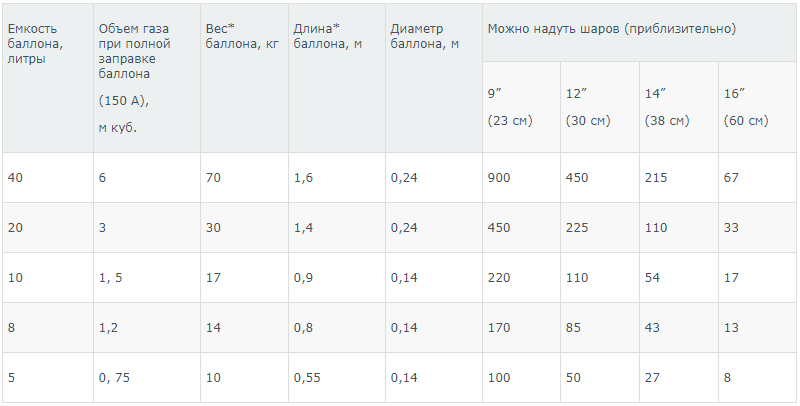
Standard cylinders, under a pressure of 150 atm, are manufactured at specialized enterprises. This pressure means that the substance is compressed 150 times, and this makes it possible to fit 150 times more gas into the container. Cylinders with a volume of 40 liters will actually hold 6000 (40×150) liters of gas. In this state, in addition to helium, nitrogen, oxygen, and various gas mixtures are stored. Helium balloons differ only in color – they are brown in color. In the production of containers, great importance is attached to the quality of the valve, which must close tightly due to the very high fluidity of the gas.
Due to the high internal pressure in the cylinder, there are often problems with measuring residues. How can you measure the amount of helium in a cylinder and determine its exact volume? A special measuring device is intended for this – a manometer, which makes it easy to determine the pressure in the tank. If the device shows a pressure of 80 atmospheres in a 10-liter cylinder, then to determine the volume of the remaining gas, the following arithmetic calculation must be made: 90 atmospheres x 10 liters = 900 liters. This number will be the residual volume of helium in the tank. This value is used to calculate the sufficiency of gas when performing a certain amount of work. For example, solving such a problem will be useful in calculating the possible number of latex balloons that need to be filled with helium. In this case, you can use the table with the following information:
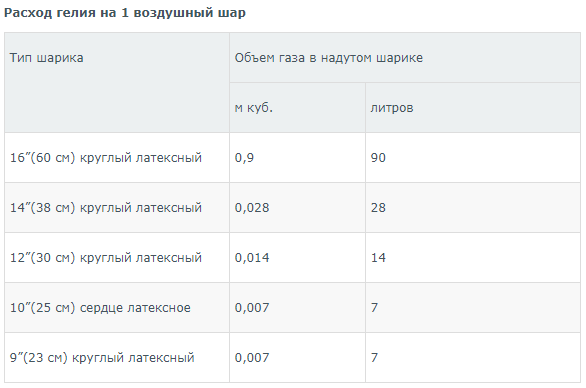
Continuing to calculate on the basis of tabular data and given that there are 900 liters of gas in the cylinder, we get the following results, in which it is not necessary to take into account the remainder:
900/90 = 10 balls of 14 inches;
900/28 = 32 balls 14 inches;
900/14 = 64 balls 12 inches;
900/7 = 128 balls 9 inches;
900/7 = 128 balls 10 inches (heart-shaped).
The information on the left side of the table showing the volume of helium in liters may seem strange to many. Indeed, it is hard to believe that a 16-inch balloon holds 90 liters of gas. However, this information is correct because in the ball, helium is also in a state of compression. The pressure in the ball is 1.5 atmospheres due to the compression of the walls.
Residual pressure
In order that the container does not have to be washed, which is associated with some material costs, air from the environment must not be allowed to enter the cylinder. To save money, it is necessary to close the valve on the cylinder immediately after the balloon stops inflating, and do not use this container anymore. To exclude the possibility of unauthorized opening of the valve in an empty cylinder, it must be marked in any way.
Use of small-capacity helium cylinders
The bulk of helium is transported in large volumetric cylinders. However, it is not always easy to transport such containers, because a 40 liter cylinder weighs almost 70 kg. In addition, it is not very convenient to work with such massive containers. Therefore, more and more smaller cylinders are used, although they also have their relative disadvantages – for example, they require more frequent refueling. This is associated with an increase in transportation costs, since containers are filled in specialized factories. Refueling cylinders at home is almost completely excluded, because this process requires special knowledge and technological equipment, compliance with safety measures.
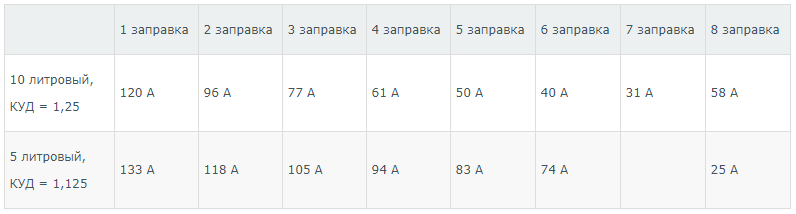
However, in principle, it is possible to fill several small specialized containers with one large cylinder. In the presence of a 5-liter container, in which the pressure is 1 atm, as well as a cylinder with a volume of 40 liters and a pressure of 150 atm, the substance can be overflowed. To do this, you need a high pressure hose and good quality sealing gaskets. In the process of overflow, the pressure in the tanks will equalize, and the gas will flow from a large cylinder to a less voluminous one. The total pressure in the connected system will be 133.33 atm, having decreased by 1.125 times (pressure reduction factor). With subsequent refueling, the pressure will also decrease.
For a standard 40-liter cylinder with a pressure of 150 atm, the following table can be compiled based on the pressure change data:
Indicators of pressure in the container after filling from a standard 40-liter cylinder with an initial pressure of 150 atmospheres